 People with cancer tend to have a low pH, which is an acidic body condition. Based on this one meager fact alone, we want to avoid an acidic body condition.
People with cancer tend to have a low pH, which is an acidic body condition. Based on this one meager fact alone, we want to avoid an acidic body condition.
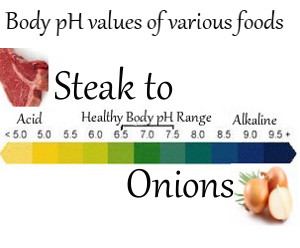
To do this we need to know what “acidic body condition” means, and we need a basic understanding of the body pH values of various foods
What exactly is an acidic body condition?
First of all, an acidic body condition is distinct from the high concentration of hydrochloric acid you need in your stomach to release vitamin B12 and magnesium from protein in food you eat.
As essential as stomach acid is to release vitamin B12 and magnesium from food, it can still be dangerous. Your body, however, is immensely clever and provides R-binders to protect newly released B12 from being denatured by stomach acid.
In addition, parietal cells in your stomach lining secrete not just hydrochloric acid but also a glycoprotein called intrinsic factor. Intrinsic factor is a must in the absorption of vitamin B12. If your parietal cells become damaged and cease making intrinsic factor, as can happen if you have h.pylori, you can no longer absorb B12 from food.
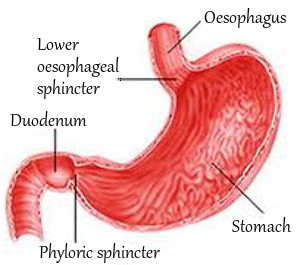
In the high acidity of your stomach, intrinsic factor has a strong affinity for the R-binders containing B12. As digesting food moves into your duodenum, the first part of your small intestine, the R-binders become partly digested by your pancreatic proteases. This allows them to release their vitamin B12.
The freed B12 is relatively safe in the more neutral, less acidic pH of your duodenum. Freed B12 and the protective intrinsic factor proceed to the lower end of your small intestine. There, you absorb the vitamin B12.
The digestion process shows that in its place high acidity is good and that to be healthy your entire body does not need a low acidic pH. In fact, the pH level of your saliva, stomach, blood, and urine, to name just a few parts of your body, are likely to be very different but equally healthy.
It is your overall body pH, most often measured using test pH strips and your urine, that indicates whether you are more or less prone to health.

Acidic foods are alkalizing
You may be tempted to think that obviously acidic foods, like lemons, are the problem, but acidic foods like lemons, grapefruit, and oranges are alkalizing within your body.

Many foods which are not as obviously acidic as citrus fruits also have an alkalizing effect; for example: avocados, broccoli and olive oil.
Cancer, as well as many diseases not as fearfully regarded as cancer, are linked to low pH, for instance, diabetes.
Dr. Atkins advised acidosis to lose lbs
The connection between acidosis, the condition of low pH in the body, and diabetes was first brought to my attention in Dr. Atkins’ books on weight loss.
Dr. Atkins explained that by eating primarily protein you can put your body into a state of acidosis, and, in a state of acidosis your body will rapidly burn fat, causing bad breath, which he felt was a minor side affect.
To be sure you reached the point of acidosis he suggested testing your urine with pH strips, which, he said, were familiar to people with diabetes.

I tried the Atkins diet and indeed rapidly lost weight, but steaks, bacon, pork chops and cheese became less and less appetizing.
At the time, as evening front desk clerk at a small Best Western here in Santa Fe, I had an opportunity to talk to a lot of people, many of whom flourished on the Atkins diet and never found steaks unappetizing.
Dr. D’Adamo’s blood type perspective
Since then I’ve read Dr. D’Adamo on the relationship of different foods to different blood types. The varying relationships explained why some people thrived on the Atkins diet, suggesting that they were the ones with type O blood.
I have type A or AB, I forget which. What I remember is that my blood type is the type which benefits from drinking coffee and eating celery. I know I don’t have type O or type B and I know that Dr. D’Adamo says people with type O benefit from eating meat.
Symptoms of low pH:
- Aging appears more rapidly
- Bladder conditions
- Body temperature lower than normal
- Cardiovascular damage occurs
- Complexion becomes pale
- Constipation
- Depression
- Diabetes
- Digestion slows
- Eyelids inflame
- Fatigue and low energy
- Fingernails thin and split easily
- Free radical damage accelerates
- Gastritis
- Gums inflamed and sensitive
- Hair is dull, ends are split, falls out easily
- Headaches
- Hormonal problems
- Immune deficiency
- Infections become more common
- Joints become painful
- Kidney stones
- Legs cramp and spasm
- Lips crack at their corners
- Muscles ache
- Skin dry, flakes and is easily irritated
- Stomach acid becomes a problem
- Stress felt more easily and more often
- Teeth loose and painful
- Ulcers of mouth and stomach
- Weight gain
- Yeast/fungal problems

Methyl-B12 1,000 mcg 100 lozenges
Low pH and low B12 affect your blood
Many symptoms of low pH are also symptoms of low vitamin B12 levels.
Both low pH and low vitamin B12 levels affect your blood, which in turn affects the amount of oxygen your tissues receive. When you have low B12 levels your red blood cells are unable to properly divide. They become too large to pass easily into your small vessels.
With low pH (high body acidity) your blood cells clump together and can’t pass easily into your small vessels.
In both cases the lack of oxygen to your cells makes you feel tired.
Healthy blood cells are kept separate from each other by their negatively charged outside and positively charged inside. In an over acidic body the acid strips blood cells of their negative charge, without which they no longer have the repelling force they need and begin clumping together.
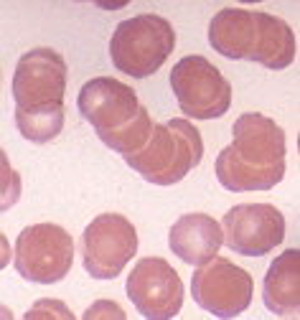
Stripped of their negative charge, blood cells clump and can’t get into small vessels.
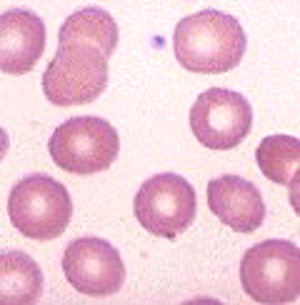
Healthy blood cells are separate from each other and move freely.
Chart: Most to Least Acidifying Foods
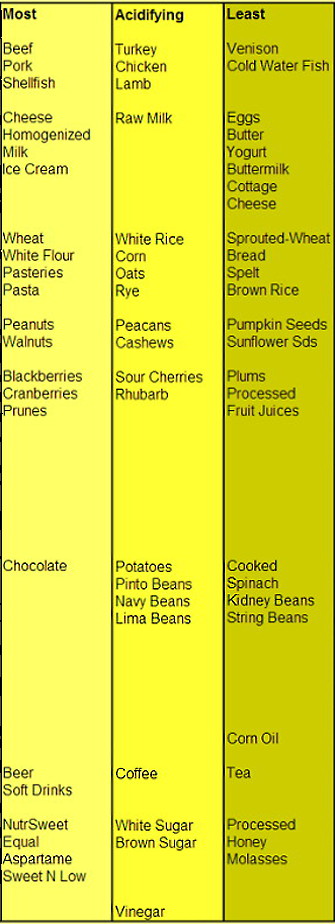
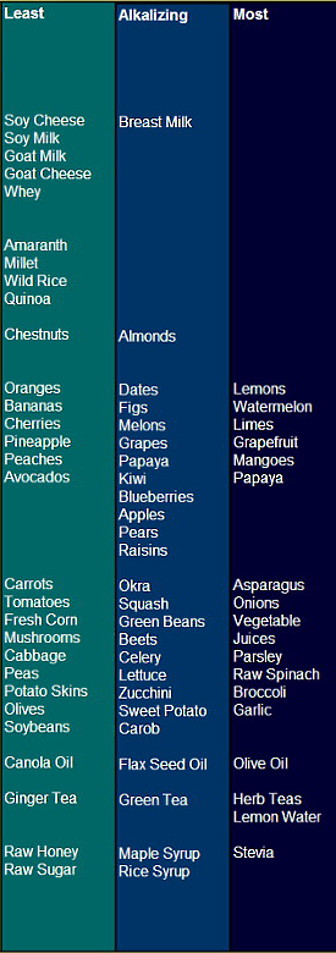
Chart: Least to Most Alkalizing Foods
Vinegar and Beau’s Lines ~
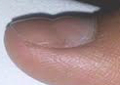 A coworker once told me she could get rid of my bad cough, but I wouldn’t like it. She said to mix equal parts of tea, lemon juice, and apple cider vinegar, add honey and drink it. It wasn’t quite as vile as it sounded, and she was right: it got rid of my cough. It also made me sleep, which I had been having trouble doing. I used the “cure” every night for weeks until I noticed that my thumbnails had horizontal grooves forming. When I stopped using the vinegar drink, the grooves smoothed out to normal.
A coworker once told me she could get rid of my bad cough, but I wouldn’t like it. She said to mix equal parts of tea, lemon juice, and apple cider vinegar, add honey and drink it. It wasn’t quite as vile as it sounded, and she was right: it got rid of my cough. It also made me sleep, which I had been having trouble doing. I used the “cure” every night for weeks until I noticed that my thumbnails had horizontal grooves forming. When I stopped using the vinegar drink, the grooves smoothed out to normal.
Some years later when I was living in hydrogen sulfide from the outdoor toilet pit under my condo I had the horizontal grooves appear once again.
At that time I couldn’t eat sauerkraut, mustard, pickles, or anything containing vinegar without my ankles and feet swelling. Only after I moved out of my condo, and after time passed, could I once again eat mustard, which I love.
Now that I know about pH, I better understand the vinegar was overwhelming my system.
Potassium keeps pH high, osteoporosis low
Every time you lift a finger or blink your eye your sodium/potassium ion pumps are putting your thoughts into action. Although we hear a lot about calories, fats and sodium, how often do we hear about potassium? I hardly ever hear “potassium,” despite its huge importance. Potassium is essential for the health of every cell in your body,
Potassium-rich foods, like fruits and vegetables, are rich in precursors to bicarbonate ions, which buffer acids in the body. The modern Western diet tends to be low in sources of alkali (fruits and vegetables) and high in sources of acid (fish, meats, and cheeses). When the quantity of bicarbonate ions is too low to maintain normal pH, the body can mobilize alkaline calcium salts from bone in order to neutralize acids consumed in the diet and generated by metabolism. Increased consumption of fruits and vegetables reduces the net acid content of the diet and may preserve calcium in bones, which might otherwise be mobilized to maintain normal pH.
Morris RC, Frassetto LA, Schmidlin O, Forman A, Sebastian A. Expression of osteoporosis as determined by diet-disordered electrolyte and acid-base metabolism. In: Burkhardt P, Dawson-Hughes B, Heaney R, eds. Nutritional Aspects of Osteoporosis. San Diego: Academic Press; 2001:357-378.
Olive oil’s high pH may balance out low pH foods
To my way of thinking, it seems highly likely that the reason olive oil with its high pH is so healthy for us is that it balances out the low pH of foods cooked in it, so that they no longer have a damaging effect.
